Interim results of a Phase 1 clinical trial based on two doses
The Canadian Press, November 10, 2020
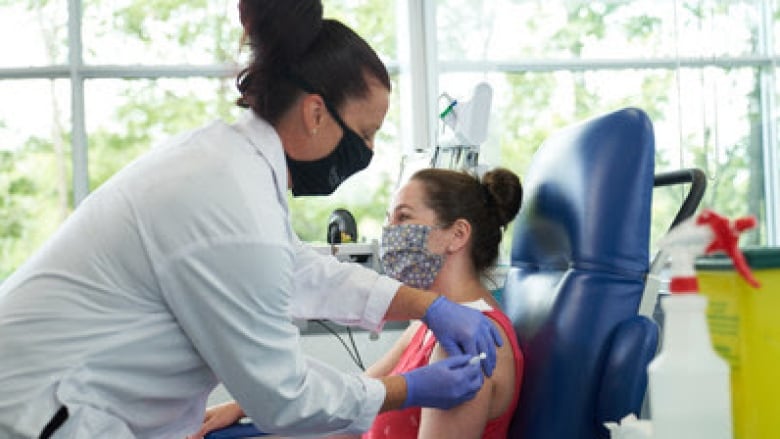
A volunteer participates in Medicago’s Phase 1 clinical trial for its COVID-19 vaccine candidate in Quebec City. (CNW Group/Medicago)
Canadian drug developer Medicago said Tuesday it has received promising early test results for its plant-derived vaccine for COVID-19.
The Quebec City-based company said interim results of a Phase 1 clinical trial found all subjects developed “a promising antibody response after two doses” of its COVID-19 vaccine candidate.
Phase 1 clinical trials include about 100 volunteers and are designed to flag any immediate safety concerns and offer an initial indication of whether the shots produce an immune response.
Medicago did not disclose full safety data and said the side effects were generally mild to moderate and short in duration. The company posted the results on a preprint server and still need to be peer reviewed by independent experts to check its validity.
The Phase 1 clinical trial was a randomized, partially blinded study of 180 healthy people aged 18-55.
Based on the Phase 1 data, Medicago plans to proceed with a Phase 2/3 clinical trial for its plant-based COVID-19 vaccine candidate, subject to regulatory approval.
World needs many vaccine suppliers, CEO says
The company said it planned to use a lower-dose version of its vaccine, along with GlaxoSmithKline’s adjuvant — a compound that enhances the body’s immune response.
The federal government has signed a deal with Medicago to secure the rights to buy 76 million doses of its vaccine. Medicago will also receive $173 million in funding from Ottawa for its vaccine research and development and for the construction of its Quebec City manufacturing facility.
Medicago’s chief executive officer, Bruce Clark, said the company is in talks with several other countries for potential deals.
“Even if you add the total number of doses that have been committed [by other companies,] you are looking at a global population of seven billion and it will require different suppliers,” Clark told Reuters.
Medicago is Canada’s most advanced domestic COVID-19 vaccine project but lags behind larger, global rivals such as Pfizer, AstraZeneca and Johnson & Johnson, which have begun late-stage trials.
On Monday, Pfizer said initial data from its large-scale trial on more than 43,000 volunteers suggested its vaccine was more than 90 per cent effective.
Pfizer’s study has not been peer reviewed, safety data hasn’t been released and infectious disease physicians say key questions such as how well the vaccine works in blocking transmission, how long it protects and who it protects still need to be answered.
With files from CBC News and Reuters